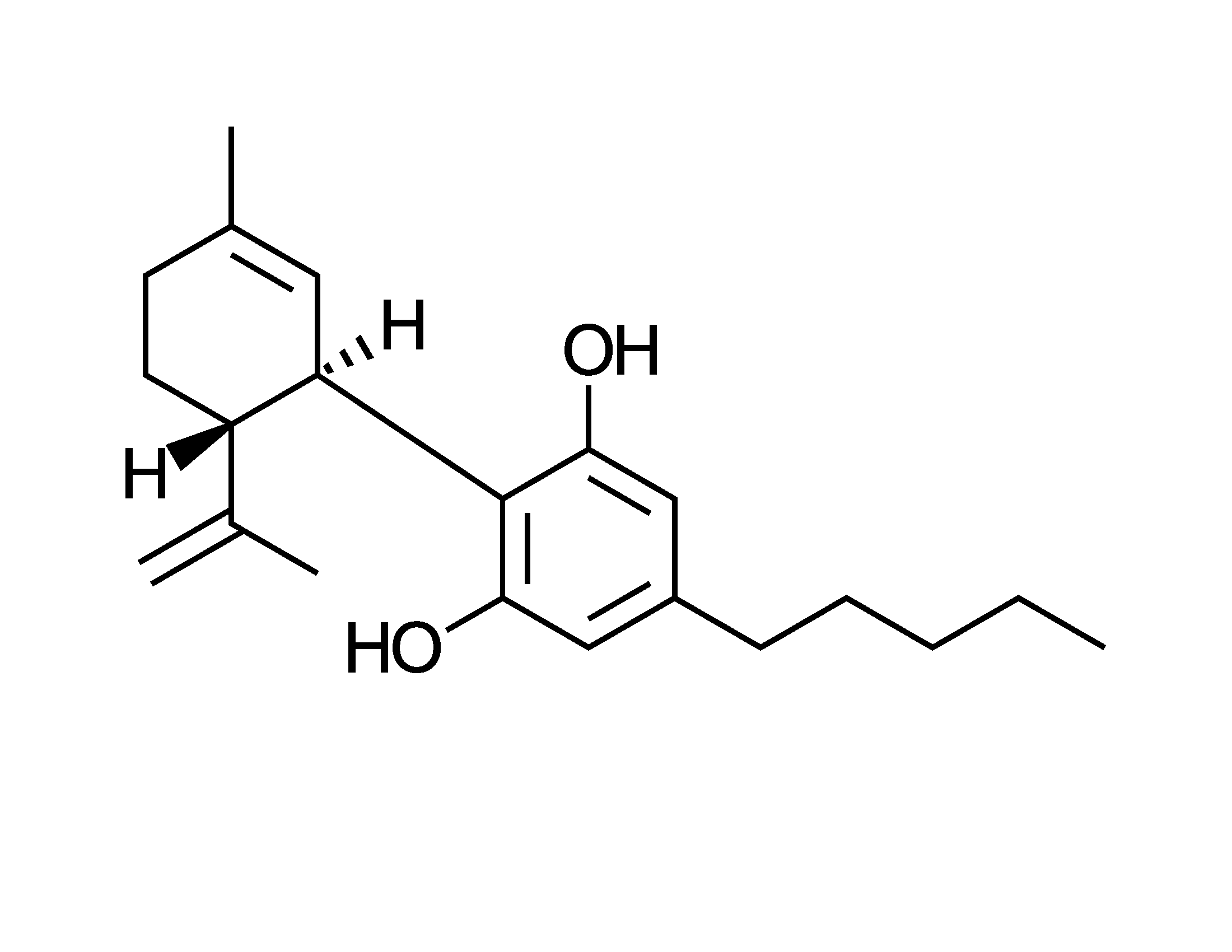
Cannabidiol is everywhere. I’ve even seen gas stations with big signs outside advertising their CBD products. But there’s an elephant in the check-out line: almost nobody lists the amount of CBD on their labels. There are reasons for that. First, the purified material is still shockingly costly. Thus, many sellers add the minimum to qualify for marketing claims: 0.1%.
But the bigger problem is stability. CBD is one of the least stable ingredients in products, and before you get your hopes up, the other cannabinoids will have similar issues. The problem is aggravated by exposure to air, to heat (such as in delivery trucks in the summer) or if it’s not in dry form. Plants overcome this by ongoing generation of the carboxylic acid derivative, and because their sludgy oils exclude air and other threats. But we don’t have the luxury of keeping the plant alive within a cream or pill. And formulating anaerobically would add cost.
You’d think that with all the groups making CBD products, stabilization would have been figured out by now. Yet in my online hunts few if any of the product teams seem to be equipped for that. In fact, the modest minority that has a chemical background tends to be on the analytical side, as opposed to focusing on the reaction chemistry. So here goes.
For the conditions in retail products a mechanistic chemist wouldn’t expect such species (i.e., terpene phenolics) to suffer much attack from acids or bases. That’s because molecules in this class have no special appetite for protons. Well, that’s not entirely true; strong acid has been reported to yield tetrahydrocannabinol (THC) as a byproduct, but consumer products are unlikely to have the necessary conditions. And the pH for tearing off CBD’s own protons is mainly above 9, which is getting fairly alkaline.
Radicals, though, that’s a different story. Bear in mind that even atmospheric oxygen is a double radical, which is one reason air is so corrosive. It’s an ocean of gas seeping into everything and looking for something to react with. By my count nearly half of CBD’s thirty hydrogen atoms (some more than others) are susceptible to being picked off by a radical. And CBD has two sites on double bonds where it practically invites radicals to plant themselves.
With a line-up like that, even if we strip out 99% of any radical source, the remaining 1% can start the ball rolling. And when they do, they transfer their own radical character to the newly formed CBD derivative. So, we’re off to the races with radical chain reactions. In other words, a trace amount of radical can initiate conversion of all the CBD to byproducts.
Next, consider that many formulas include certain agents that are radical formers. Here we have things like peroxides, sulfhydryl groups (I’m looking at you, proteins and cysteine), metallic nanoparticles, certain metal salts, and the like. And consider paraben preservatives, which exist in ionic form under use conditions and react with air to form radicals.
In theory we could beat the problem by shielding CBD. Oil-in-water emulsions might help. Embedding CBD in a gummy matrix might help. And there are host-guest options such as cyclodextrins to tuck CBD into a tube or some other shell. But the bottom line is, CBD isn’t like some rock that you add and find it still there when you come back. It gets transmogrified.
Food for Thought
Anonymous, “Cannabidiol,” in the federal PubChem database, entry no. 644019. https://pubchem.ncbi.nlm.nih.gov/compound/Cannabidiol